www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
Why does the pepper take flight?

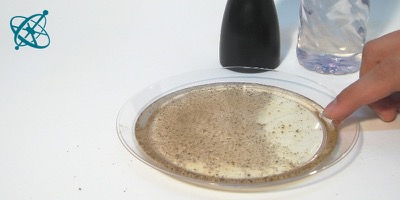
To see this, sprinkle some pepper on water.

Get a tiny bit of detergent on your fingertip…
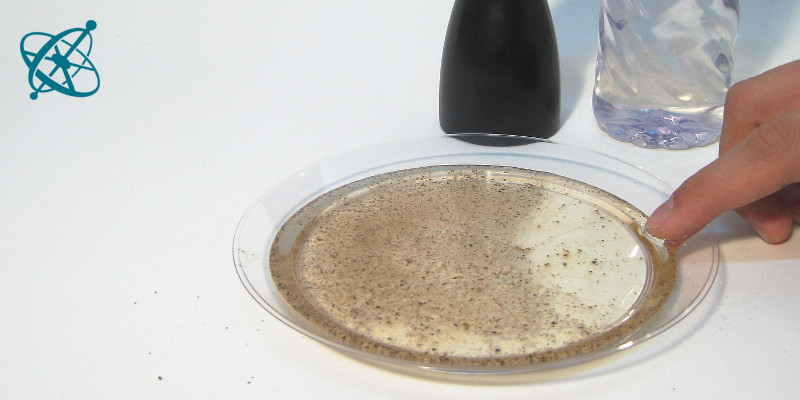
…and just touch the water.
Fleeing pepper
Surfactants (e.g. detergents) tend to build a monomolecular film on a water surface. While a monomolecular layer is hard to observe with the naked eye, in this experiment it can be 'seen' pushing aside pepper on the water surface.
Surfactants, like e.g. dishwashing detergents, spread on the water surface.
Provide experimental evidence for a scientific argumentation.
Plastic dishes
Pepper
Water
Hand out plastic dishes to your students, let them fill them with water and pass a pepper mill so that students can sprinkle pepper onto the water. Then pass around a bottle of common detergent and ask students to put as little as possible of it onto their finger.
Prepare a bucket you can pass after the experiment to collect the materials.
1. What happens if you touch the water with the detergent on your finger?
2. How do you proof this? Think of experiments that provide scientific evidence for or against your hypothesis.
Is the pepper pushed aside by the reduction of surface tension?
› No, the reduction of surface tension would make the pepper sink, not move to the side. This can be shown by sprinkling pepper on water mixed with detergent.
Is there a repelling force between pepper and detergent?
› No, just holding the detergent close to the pepper (without touching the water) doesn't move it.
Does the effect depend on the quantity of detergent used?
› Yes, the more detergent comes in contact with the water, the larger the area that becomes 'freed' from pepper.
Does the effect come from the detergent diffusing in the water?
› By dropping ink into the water it becomes clear that the diffusion is far too slow to explain the pepper's quick retraction.
Where does the detergent go?
› It spreads on the water surface, pushing aside the pepper.
As the surfactant molecules spread on the water surface – in form of a thin film with their water-soluble parts downwards – they quickly push the pepper aside. Please note that the 'fleeing' of the pepper is NOT caused by a reduction of the surface tension. If that was the case, the pepper would simply sink.
With well-paced questions, you can stimulate your students to exclude incorrect explanations and try to find experimental evidence for their hypothesis. As much as your time allows, encourage them to conduct the experiments they suggest and discuss what conclusions can – and cannot – be drawn from them.