www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.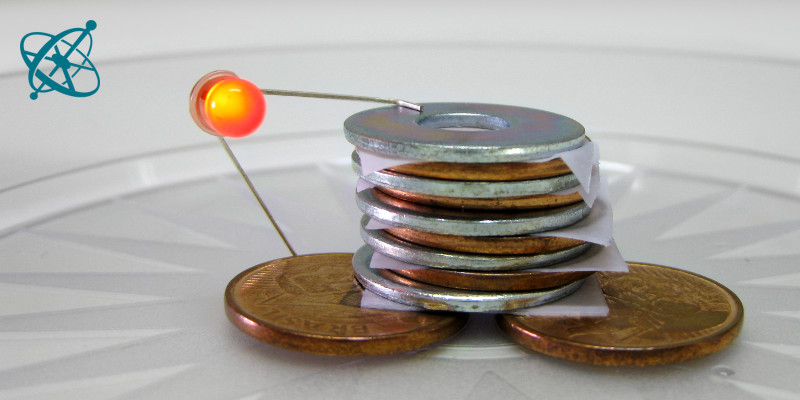
Build your own battery!

Soak the paper in vinegar.

Start your pile with a base of copper coins…

…add a piece of paper…

…and a zinc-coated washer.

Then repeat with another copper coin…

…a paper…

…and a washer until all the coins and washers are used.
Voltaic pile
For many years, Luigi Galvani and Alessandro Volta had an academic dispute about electricity. Luckily for us today, they based their respectful exchange of arguments on experimental evidence. As a result, both of them could make important contributions to science. Volta, for instance, argued for his theory by inventing the battery!
Within minutes, your students will have build their own Volta pile. And you will love to see their excitement, when their simple stack generates enough electricity to power a LED.
Understand how a battery generates electric energy.
Zinc-plated (galvanized) washers
Paper
Vinegar
Red LED
Plastic dish
To save time for more important things, it is recommended to pack for each group a plastic bag with 7 copper coins, 5 zinc-coated washers, at least 5 pieces of paper roughly the size of the coins, and one LED.
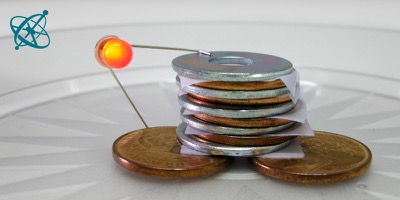
Before handing out the material, explain with a drawing, or by using the photos above, how to stack the pile and how to probe the 'battery' with an LED.
Pour a few drops of vinegar on the papers. To build one cell of your battery, start with a base of copper coins, add a piece of wet paper and then a zinc-plated washer. Stack 4 or 5 of such cells onto each other: coin–paper–washer–coin–paper–...–washer. Then hold the LED so that the longer leg touches the coins at the base and the shorter leg the washer on the top.
1) Where does the energy come from that generates the electric current and makes the LED shine?
When the coins, papers and washers are stacked correctly, the LED will give a soft glow for a pile of 4 cells and a brighter glow for a pile of 5 cells.
The energy, which the LED transforms into light, comes from a redox reaction. It can be understood as a 'trade' in electrons between the zinc, which prefers to be Zn2+ ions in solution (in this experiment the vinegar), and the Cu2+ ions in the oxid layer on the coin, which prefer to be just copper without charge. The zinc at the washer is 'oxidized'
Zn → Zn2+ + 2e-
while the copper ions in the copper oxide are 'reduced' to copperCu2+ + 2e- → 2Cu
When all the copper oxid has been reduced, the reaction continues with the reduction of hydrogen ions, generating hydrogen gas:2H+ + 2e- → H2
These reactions take place in the proximity of the coin and washer surfaces. Where a coin lies on a washer, the 'surplus' electrons from the washer can easily travel to the coin, thereby creating an electric potential. By connecting the upmost washer with the coins at the base, the electric circuit is closed and all redox pairs that want can exchange electrons.